(1)Introduction: The families alcohols, ethers, phenols, and thiols may be considered in the context of water as follows: water = H-O-H; alcohol = R-O-H, where one hydrogen atom of water is replaced by a hydrocarbon functional group; ether = R-O-R, where both hydrogen atoms of water are replaced by a hydrocarbon functional group; phenol = phenyl-O-H, where on hydrogen atom of water is replaced by a benzene ring; thiol = H-S-H, where the oxygen atom of water is replaced by sulfur. Note that R- designates any alkane functional group, for example CH3- is methyl, etc.
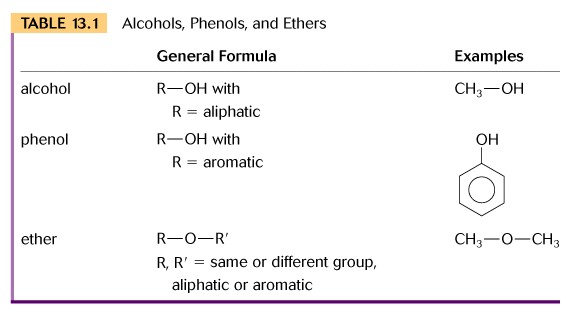
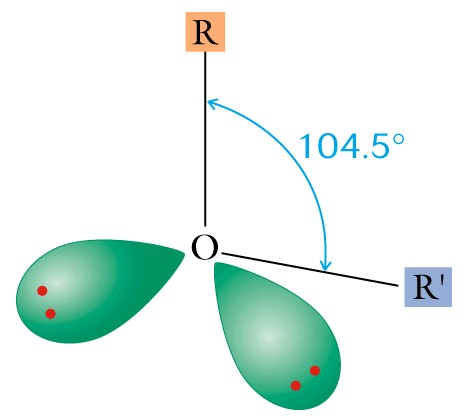
(2) Properties: The oxygen atom in each family member is SP3 hybridized to form sigma bonds, and the difference in electronegativity between oxygen and hydrogen produces a delta charge on the oxygen and the hydrogen atoms.
- In alcohols this allows hydrogen bonding between the molecules and this will influence the physical properties just like we saw in water.
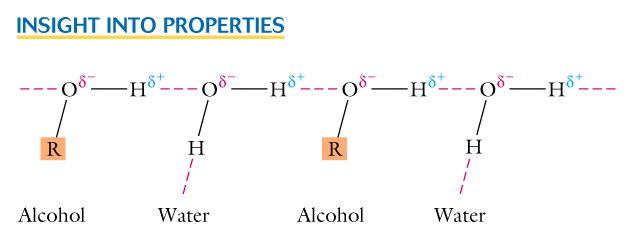
- In ethers there is the strong electronegative center (oxygen) , but no hydrogen atom. Thus ethers will not hydrogen bond among themselves, but with any other molecule where hydrogen is bonded to oxygen or another strong electronegative center.
- Thiols contain sulfur rather than oxygen and are considered sulfur analogs of water.
The difference in the value of electronegativity between hydrogen and
sulfur is NOT large enough to make this functional group polar, and thiols
do not form hydrogen bonds. The cells of the liver contain enzymes capable
of adding a hydroxyl group to non-soluble, toxic molecules so that they
become water soluble and can be carried in the blood to the kidneys for
excretion.
(3) Nomenclature of alcohols and ethers. In the IUPAC system for naming alcohols the ending (-ol) replaces the ( -e) of alkane. In addition, primary, secondary, and tertiary denotes the presence of two, one, or no hydrogen atoms bonded to the carbon containing the hydroxyl group. Alcohols may also contain more than one hydroxyl group, a polyhydroxyl alcohol that you must learn is glycerol or 1,2,3-propanetriol.
Write the structural formula for this important building block of many lipids.
For naming ethers, select the longest carbon chain as the parent compound
and name group as the alkoxy substituent, (-oxy) followed by name of the
other alkane group in the molecule. The common name uses the
word ether after the two alkanes have been named, i.e. dimethyl ether.
(4) Some special ethers - the most important ethers among biological molecules are the heterocyclic ethers, such as furan and pyran. Furan contains four carbons with two double bonds and one oxygen in a five-member ring, and pyran contains five carbons with two double bonds and one oxygen in a six-member ring.
Write the structure of each of these ethers and commit them to memory.
Later we will see their importance in the structure of sugars, where
the hydroxyl and carbonyl functional groups occur in the same molecule
and an intramolecular nucleophilic addition reaction occurs to form the
ring or cyclic structure of either pyran or furan.
(5) Phenols - these are highly stable enols via the gain in resonance
stabilization of the ring structure that we saw in the benzene ring (remember
the pi cloud?), and they differ from alcohols in being more acidic.
(6) Thiols - these are considered sulfur analogs of alcohols and
their physical properties differ from alcohols because of the lack of
polarity. Thus this functional group (-SH) does not engage in hydrogen
bonding. Their most important bonding occurs in proteins where the presence
of two or more amino acids containing this functional group allows the
formation of a covalent sulfur-to-sulfur bond via the oxidation/reduction
reaction of (-SH + HS-) to form the (-S-S-) linkage. This important
covalent linkage allows proteins to assume unique shapes, and is also
used to covalently link together individual polypeptide chains when the
protein consists of two or more of such subunits. A good example is the
insulin molecule, and you should look up the structure of this important
hormone.
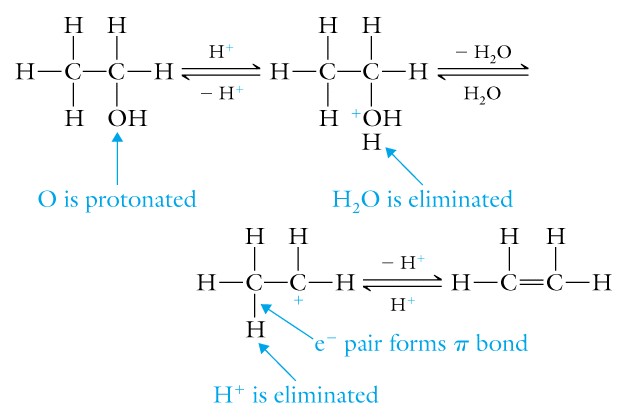
(7) Chemical reactions of alcohols are: dehydration to alkene -
similar to alkene hydration, but in the reverse; oxidation/reduction of
primary alcohols to the corresponding aldehyde, secondary alcohols to
the corresponding ketone, and tertiary alcohols without a hydrogen are
not reactive (remember that biological oxidations involve the loss of
electrons or hydrogen and are always coupled to reductions or the gain
of electrons or hydrogen, the enzyme is termed a dehydrogenase and works
with a coenzyme like NAD or NADP ). Thus if you oxidize an alcohol with
a dehydrogenase enzyme, the coenzyme in the oxidized form is converted
into the reduced form.